Overview
Brazil’s standards for registration of biological products is outlined in regulation RDC No. 55/2010. The guideline provides two regulatory pathways for follow-on biological products: a comparative pathway and an individual development pathway.
What requirements do foreign companies abide by for importation and marketing products into Brazil?
Because of the nature of the products, Biologics can only be imported and marketed in Brazil if the foreign company :
- Establishes a local Brazilian manufacturing unit or local office
OR
- Appoints a Brazilian legal representative who is able to market the products.
Brazilian Local Representative :
The representative must also be authorized by the Brazilian authorities to import and distribute medical products. The pharmaceutical company will declare the value of the shipment before sending the products and will provide its legal representative with all the documents as they will need them to support the declarations to Custom.
Is local manufacturing and/or Clinical trials required in Brazil if my product already has foreign data?
No, local manufacturing and local clinical trial is not required as the foreign data is acceptable in Brazil
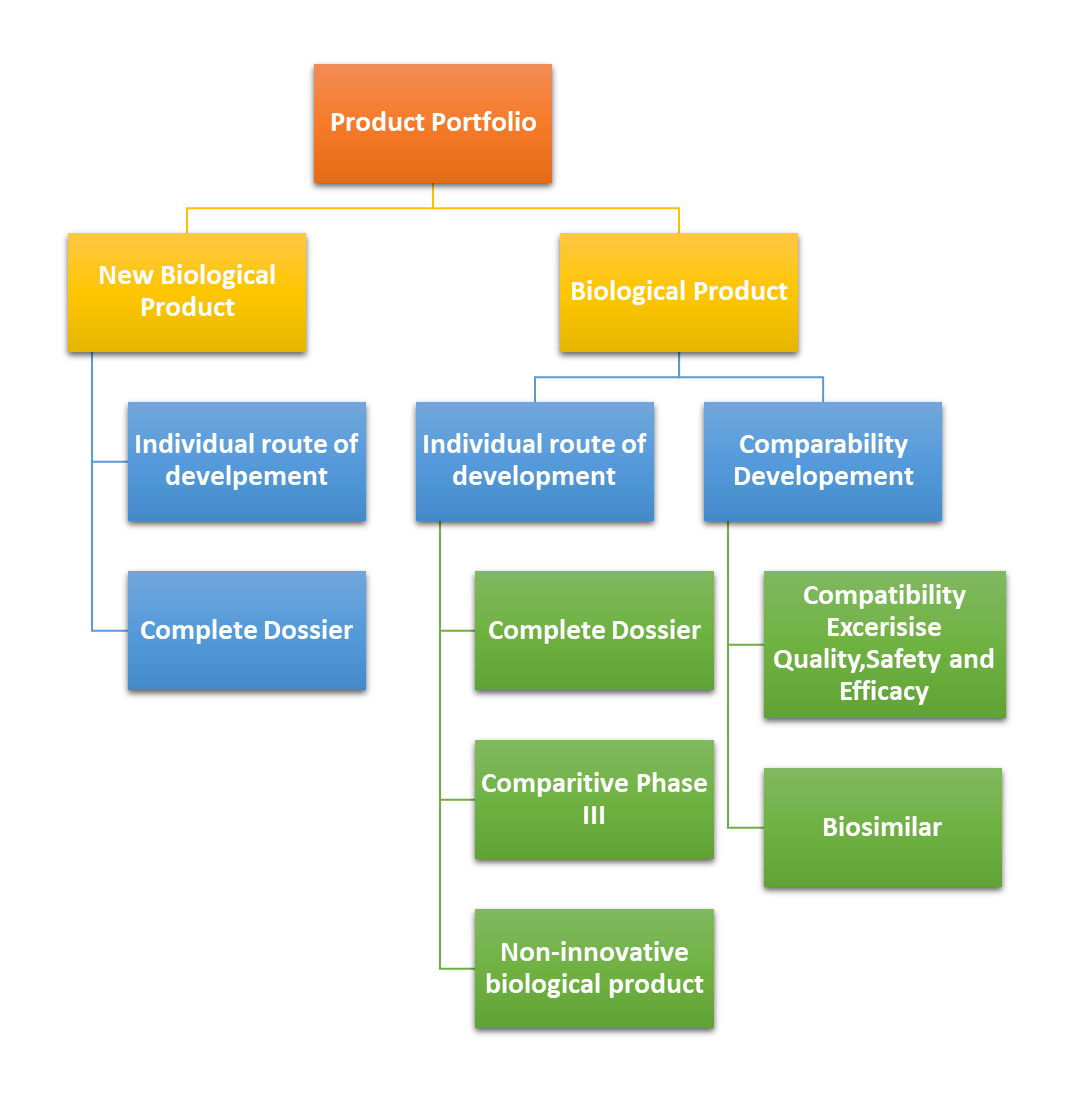
What are the different routes available in Brazil through which we can register biologic products?
There are several Product Registration pathways available for Biologic products in Brazil, including;
- New Product
- Individual Development Pathway
- Comparability Development
Individual Development Pathway:
This regulatory route that can be used to register a biological product, in which there is need to present full data on the development, production, quality control and nonclinical & clinical data to demonstrate the quality, efficacy and safety of the product. Production and Quality Control data must meet the quality standards already established for the product to be registered.
Comparability Pathway:
The comparability pathway regulatory route that can be used to register a biological product, in which the comparability exercise in terms of quality, efficacy and safety was used between the developed product and the compared biological product. Extrapolation of safety and efficacy data for other therapeutic indications of the biological products registered through the comparability pathway will be established through specific guidelines.
Regulatory Pathway based on Biologic Classification
What Pathways exist for fast track approval of biologic / biosimilar products?
Different approaches need to be discussed with health authorities. In general following products can be evaluated for possibility of fast track:
- Specific Vaccines
- New Products
- Brand new generics
- if there are not enough drugs available to treat the disease
Timelines:
Once the application is submitted, ANVISA takes about 120-165 (+3 months administrative bureaucracy possible) days in Brazil for review of the dossier. In case of queries the duration may be extended.
Therefore, total approval timelines from the time of submission could be about 6-8 months. In cases where priority review of application could be enabled (such as some listed drugs, select vaccines etc.) the registration and approval timeline could be reduced.
About Global Regulatory Partners:
Global Regulatory Partners Inc, (GRP) is an American company that provides regulatory affairs, clinical, quality and safety services to medical devices and pharmaceutical companies globally. GRP headquarters is located in Massachusetts USA and its main affiliates are located in China, Japan, Brazil, Mexico and Argentina. GRP helps many life science companies register their products in different countries in compliance with local regulations.. To learn more, please contact us at info@globalregulatorypartners.com
More Resources:
https://globalregulatorypartners.com/countries/latin-america/brazils-anvisa/
GRP Presentation. Drug Registration in Brazil.